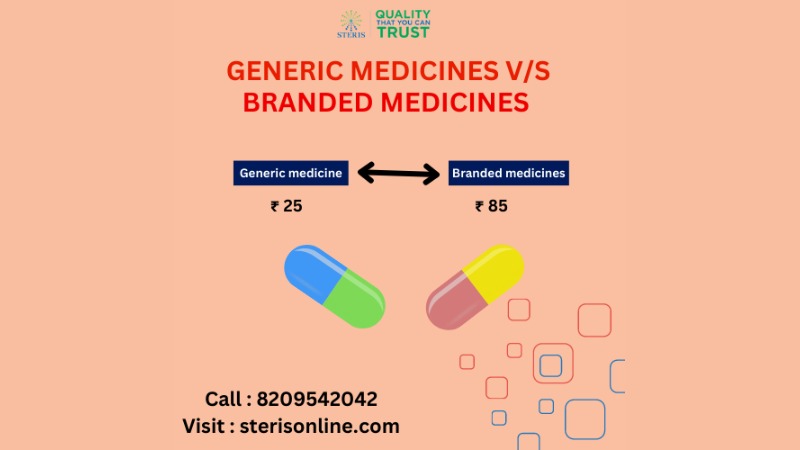
GENERIC VS BRANDED MEDICINES
Jun 13, 2023
Generic and branded medications are two types of pharmaceutical products that differ primarily in their names, manufacturers, and pricing. Here's a comparison of generic and branded medicines:
Name: Brand-name drugs are given a specific brand name by the pharmaceutical company that develops and markets them. Generic drugs, on the other hand, are sold under their chemical or generic name, which is based on the drug's active ingredient.
Manufacturer: Brand-name drugs are developed, patented, and manufactured by the pharmaceutical company that originally researched and developed the medication. Generic drugs are produced by other manufacturers once the patent protection of the brand-name drug expires. Multiple generic manufacturers may produce the same generic medication.
Pricing: Branded drugs are generally more expensive than generic drugs. This price difference can be attributed to the costs associated with research, development, clinical trials, marketing, and brand promotion. Generic drugs are typically more affordable because the manufacturers don't have the same research and development expenses and can compete on price.
Appearance: Brand-name drugs often have unique colors, shapes, and markings, serving as a part of their brand identity. Generic drugs, while having the same active ingredient, may have different colors, shapes, or markings, depending on the manufacturer. However, generic drugs must still meet the same quality and efficacy standards.
Patent Protection: Brand-name drugs are protected by patents, which give the manufacturer exclusive rights to produce and sell the medication for a specific period (usually 20 years). Once the patent expires, other manufacturers can produce generic versions of the drug, which creates competition and leads to lower prices.
Equivalence: Generic drugs are required to be bioequivalent to the brand-name drugs. This means they have the same active ingredient, strength, dosage form, route of administration, and intended use. They must demonstrate similar safety and efficacy profiles through rigorous testing and meet regulatory standards set by authorities.
Perception: There may be differences in how brand-name and generic drugs are perceived by consumers. Brand-name drugs may be associated with trust and familiarity due to their extensive marketing and brand recognition. However, generic drugs are regulated and held to the same standards as brand-name drugs, ensuring their safety and effectiveness.
It's important to note that both generic and brand-name drugs can be equally effective in treating medical conditions. The choice between them may depend on factors such as cost, availability, healthcare provider's preference, and individual patient circumstances
For further info please contact : contact@sterispharma.com
Call : 6377716668 / 8955945010
Visit : sterisonline.com
Recent Post

Calcium Citrate Malate Vitamin D3 Relief: Chondroflex Joint Pro.
Glimepiride IP 0.5mg Tablet: Up to 30% Discount Available at Steris Healthcare.

VELAXFINE XR 75: Advanced Relief with Venlafaxine Hydrochloride 75 mg for Depression & Anxiety.

Venlafaxine 37.5mg Tablet | VELAXFINE XR 37.5 from Steris Healthcare Pvt Ltd.

Glidapa Biso: Dapagliflozin 10mg and Bisoprolol Fumarate 10mg Tablet.
Tadalafil गोली: मिलीग्राम उपयोग, साइड इफेक्ट, and Price.

Dapoxetine Safe and Effective In Treating Premature Ejaculation.
Allium Cepa Extract, Heparin Sodium & Allantoin Gel: Advanced Scar Healing & Skin Repair Solution
Clobetasol Propionate, Neomycin Sulphate & Miconazole Nitrate Cream

Diclofenac Gel Enhanced with Menthol and Linseed Oil for Quick Comfort`